- Freudenberg Medical manufactures innovative silicone monitoring belt Bambi Belt for premature babies
- Initial studies in hospitals a success
Weinheim, November 16, 2021. November 17 is World Prematurity Day. A World Health Organization report states that worldwide roughly 15 million babies are born pre-term each year. Oftentimes, they spend up to 42 days in a neonatal intensive care unit where their vital signs can be kept under strict medical observation. Since monitoring electrodes and a separation from the mother can lead to discomfort and stress, half of all pre-terms go on to develop concentration problems and cognitive impairments. In 2008, pediatrician and neonatologist, Sidarto Bambang Oetomo, came up with an idea for a skin-friendly monitoring belt. Today, he runs the Bambi Belt enterprise in Eindhoven, the Netherlands, with his son, Fabio Bambang Oetomo, and 13 employees. “It’s been a long journey. But a product that improves the lives of so many premature babies is worth every effort,” says Fabio Bambang Oetomo. To create the first prototypes, the Freudenberg Medical team were on hand to support in the belt’s manufacture with material expertise as well as medical technology process and product knowledge. Small series and large quantities are also planned.
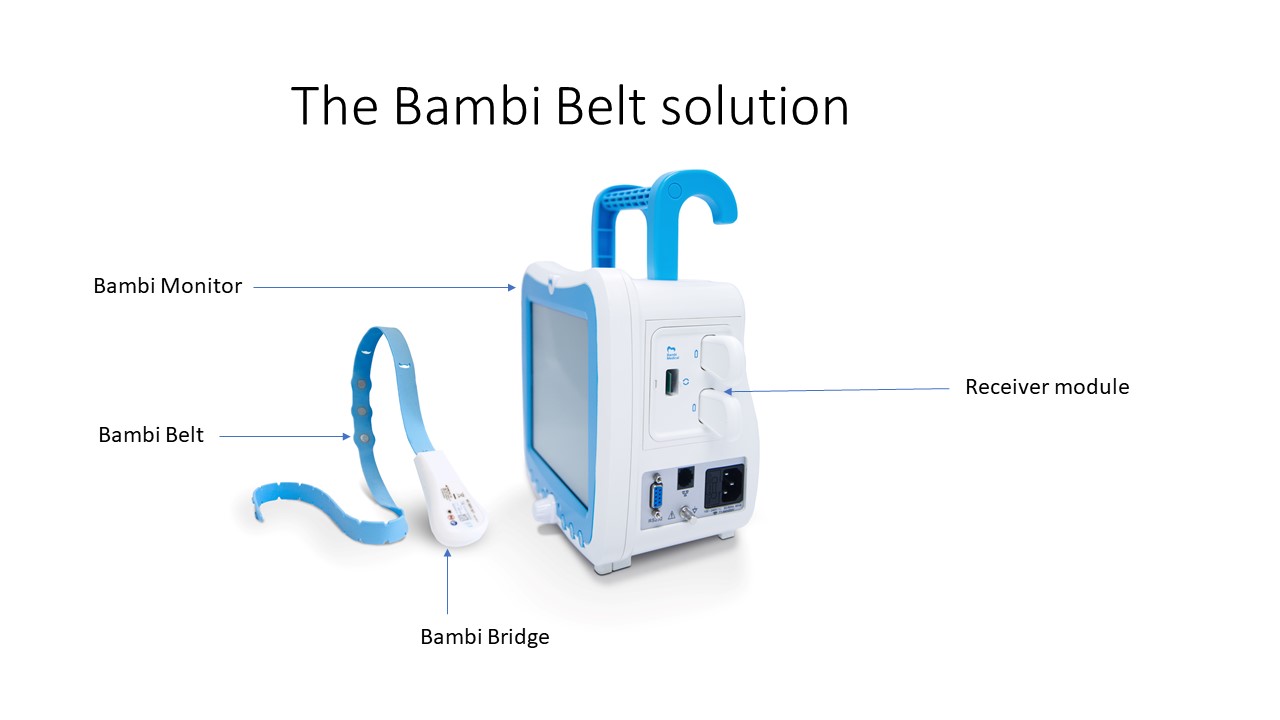
Being cable-free, the innovative Bambi belt allows parents to remove their premature baby from an incubator without causing injury to their baby’s skin. The belt itself is formed from a narrow band placed around the baby’s chest. Sensors send signals to a portable monitor. For premature babies weighing a mere 500 or 600 grams whose skin is still too sensitive for adhesive electrodes, the band makes it possible to easily monitor the heart muscle’s electrical activity and measures respiration activity. Prior to this, in the absence of adhesive-free electrodes on the market or electrodes specifically developed for sensitive skin, a patch on the baby’s foot had to be used to monitor the heart rate and oxygen content in the blood of such babies while in hospital – often leading to injury or infection.